PolyTetraFluoroEthylene - PTFE
Production Process of PTFE Polymer
Basic Production Process of PTFE Polymer:
The Manufacturing of PTFE Polymer/ Resin is basically carried out in two stages. First, TFE Monomer is generally manufactured by synthesis of Calcium Fluoride (Fluorospar), Sulphuric Acid & Chloroform & later polymerisation of TFE is carried out in carefully controlled conditions to form PTFE. Due to presence of stable & strong C-F bonds, PTFE molecule possesses outstanding chemical inertness, high heat resistance & remarkable electrical insulation characteristics; in addition to excellent frictional properties.

Preparation of Monomer – TFE Monomer:
Tetrafluoroethylene was first
prepared in 1933. The current commercial synthesis are based on fluorspar, sulphuric
acid and chloroform.
The reaction of fluorspar (CaF2) and sulphuric acid yields hydrofluoric
acid.
 CaSO4 +
2HF
CaSO4 +
2HFPreparation of monochlorodifluoromethane and Tetrafluoroethylene (TFE): Reaction of hydrofluoric acid with Chloroform yields monochlorodifluoromethane
CHCl3 + 2HF CHClF2 + 2HCL
CHClF2 + 2HCLBoiling point of monochlorodifluoromethane is -40.8°C. And also used as a refrigerant. To prepare the monomer monochlorodifluoromethane pyroleted, for example, by passing through a platinum tube at 700°C.
 CF2 = CF2 + 2HCL
CF2 = CF2 + 2HCLCompounds produced during pyrolysis including some highly toxic ring structures.
Purification of TFE:Pure monomer is required for polymerisation. If impurities are present it will affect the final product. The gas is first scrubbed to remove any hydrochloric acid and then distilled to separate other impurities.

Pure uninhibited Tetrafluoroethylene can polymerise with violence, even at temperatures initially below that of room temperature. A silver-plated reactor, quarter-filled with a solution consisting of 0.2 parts ammonium persulphate, 1.5 parts
borax and 100 parts of water, and with a pH of 9.2. The reactor was closed; evacuated and 30 parts of monomer were let in. The reactor was agitated for one hour at 80°C and after cooling gave an 86% yield of polymer.
PTFE is made commercially by two major processes, one leading to the so called 'granular' polymer and the second leading to a dispersion of polymer of much finer particle size and lower molecular weight. One method of producing the latter involved the use of a 0.1°% aqueous disuccinic acid peroxide solution. The reactions were carried out at temperature up to 90°C.
Another Methods :- Decomposition of TFE under the influence of an electric arc.
- Polymerisation carried out by emulsion method using peroxide initiators e.g. H2O2 (Hydrogen peroxide) and ferrous sulphate. In some cases oxygen is used as initiator.
All above reactions are highly exothermic and must be control to avoid violence explosions.
Structure and Properties of PTFE: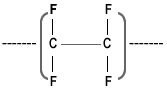
The chemical structure of PTFE is linear polymer of C– F2 – C– F2 without any branch & the outstanding properties of PTFE are associated strong & stable Carbon – Fluorine bond.
Polytetrafluoroethylene is a linear polymer free from any significant amount of branching. Whereas the molecule of polyethylene is in the form of a planar zigzag in the crystalline zone this is sterically impossible with that of PTFE due to the fluorine atoms being larger than those of hydrogen. As a consequence the molecule takes up a twisted zigzag with the fluorine atoms packing tightly in a spiral around the carbon-carbon skeleton. A complete turn of the spiral will involve over 26 carbon atoms below 19°C and 30°C above it there being a transition point involving a 1% volume change at this temperature. The compact interlocking of the fluorine atoms leads to a molecule of great stiffness and it is this feature which leads to the high crystalline melting point and thermal form stability of the polymer.
The intermolecular attraction between PTFE molecules is very small, the computed solubility parameter being 12.6 (MJ/m3)1/2The polymer in bulk does not thus have the high rigidity and tensile strength which is often associated with polymers with a high softening point. The carbon-fluorine bond is very stable. Further, where two fluorine atoms are attached to a single carbon atom there is a reduction in the C–F bond distance from 1.42 A to 1.35 A. As a result bond strengths may be as high as 504 kJ/mole. Since the only other bond present is the stable C–C bond, PTFE has a very high heat stability, even when heated above its crystalline melting point of 327°C. Because of its high crystallinity and incapability of specific interaction, there are no solvents at room temperature. At temperatures approaching the melting point certain fluorinated liquids such as per-fluorinated kerosene will dissolve the polymer.
The properties of PTFE are dependent on the type of polymer and the method of processing. The polymer may differ in particle size and/or molecular weight. The particle size will influence case of processing and the quantity of voids in the finished product whilst the molecular weight will influence crystallinity and hence many physical properties. The processing techniques will also affect both crystallinity and void content.
The weight average molecular weights of commercial polymers appear to be very high and are in the range 400000 to 9000000. ICI report that their materials have a molecular weight in the range 500000 to 5000000 and percentage crystallinity greater than 94~ as manufactured. Fabricated parts are less crystalline. The degree of crystallinity of the finished product will depend on the rate of cooling from the processing temperatures. Slow cooling will lead to high crystallinity with fast cooling giving the opposite effect. Low molecular weight materials will also be more crystalline.
It is observed that the dispersion polymer, which is of finer particle size and lower molecular weight, gives products with a vastly improved resistance to flexing and also distinctly higher tensile strengths. These improvements appear to arise through the formation of fiber-like structures in the mass of polymer during processing.



